Maryland Hospital Association, in collaboration with the Hospital Preparedness Program and the Maryland Department of Health (MDH), is actively monitoring potential IV fluid supply chain shortages due to the closure of Baxter International’s North Cove Plant, which supplies 60% of the country’s IV solutions. The plant shut down following flooding caused by Hurricane Helene.
MHA will continue to keep this page updated and the field informed about this ongoing issue. For questions, contact mha@mhaonline.org.
MHA Member Alerts and Resources for Members
Latest Updates
2024 Updates
- More than 12 million units of product from international facilities have been expedited into the U.S. from China, Spain, Canada, South Korea, and the UK, with the assistance of the U.S. Government and aviation partners, FDA, Dec. 27, 2024
- Baxter Expects Additional IV Solutions Production Lines to be Resumed by End of Month, Dec. 5, 2024
- AHA, Baxter Announces Expiration Dating Extensions for Certain Products Related to IV Solutions Disruption, Oct. 28, 2024
- AHA Special Bulletin: Baxter Provides Updates on Efforts to Increase Access to and Supply of IV Solutions, Oct. 24, 2024
- Fact Sheet: HHS Continues Taking Action to Increase Access and Supply of IV Fluids Following Hurricane Helene, Oct. 18, 2024
- Maryland Department of Health (MDH) Update IV Solution Conservation for Clinicians, Oct. 15, 2024
- CDC Health Advisory, Oct. 12, 2024
- AHA Podcast: The Critical Need for Conservation Plans in the Face of Supply Shortages
Previous Updates
Administration, Baxter Officials Provide Updates on IV Solution Supplies, Oct. 10
Baxter establishes dedicated email address for hospitals to contact for supply issues
On a call Oct. 10 hosted by Department of Health and Human Services Secretary Xavier Becerra, officials from the federal government and Baxter provided updates on the ongoing efforts to address the IV solution supply disruptions caused by the temporary closure of a Baxter manufacturing plant in North Carolina.
Among other highlights:
- Baxter officials announced that they have set up a dedicated email address that will be active on Oct. 11 that hospitals can use to contact the company with questions related to the IV solution situation, particularly if they are in dire need of product. The email address is hurricanehelenesupport@baxter.com.
- Baxter officials said they would provide updates about the situation on their dedicated webpage on Mondays and Thursdays.
- Baxter acknowledged that last week direct customers received 40% allocation of the various products, but that distributors were given a 10% allocation because Baxter was aware many distributors had additional product in storage that could be distributed. Baxter announced that going forward, distributors would receive the same allocation as direct customers.
- FDA instructed hospitals to hold onto expired products. FDA anticipates that it will extend the shelf life of some products by working with manufacturers.
- FDA is working to temporarily import some products in shortage to help meet patient needs. In this situation, FDA is carefully assessing the overseas product for quality, making sure that it is safe for U.S. patients. Please see the FDA webpage and table for more information.
- FDA clarified that the 1-mile rule is no longer in effect. Hospitals and health systems can distribute these products among their facilities without concern for the distance.
In addition, B. Braun, which is another major manufacturer of the kind of solutions made in the Baxter plant that is offline, today said its Daytona Beach IV solutions manufacturing site and distribution center were not seriously impacted by Hurricane Milton and will resume operations as planned on Friday, Oct. 11. “To safeguard the supply of finished products, we worked closely with the federal government, specifically the Administration for Strategic Preparedness and Response (ASPR), to leverage their resources which allowed us to move IV solutions inventory from our Daytona Beach distribution center to a secure, temperature-controlled facility north of Florida,” the B. Braun statement said.
Baxter to Increase Allocations for IV Solution Supplies to Hospitals Effective Oct. 9
On Oct. 8 on a call hosted by the Department of Health and Humans Services, Heather Knight, Baxter executive vice president and group president of medical products and therapies, announced that Baxter will be changing allocation for some IV solution supplies from 40% to 60% effective Wednesday, Oct. 9. In addition, Baxter expects to be at 70% by the end of October and 90% to 100% by the end of the year. We anticipate Baxter will notify customers later tonight or tomorrow, and we will provide updates as they become available. The AHA appreciates our ongoing partnership and collaboration with Baxter and HHS to help mitigate the impact to patients.
Other Resources for Members
(This list will be updated as new resources are available.)
- FDA Information and Updates on CDER
- MHA Member Alert – IV Solutions Shortage Update, Oct. 8, 2024 (Members Only)
- Maryland Hospital Association (MHA) SBAR: IV Fluid Supply Chain Shortage, Oct. 4, 2024
- MDH IV Solution Conservation Letter for Clinicians, Oct. 3, 2024
- MIEMSS IV Fluid Shortage – Conservation Letter, Oct. 4, 2024
- ASHP Fluid Shortages – Suggestions for Management and Conservation
- ECRI Supply Chain Report
American Hospital Association
Baxter International Updates
Resources from Vizient
- Vizient Drug Shortage Alerts
- Adult & Pediatric IV Push Medication Reference, June 2023
- Drug Shortage Stewardship, Oct. 25, 2022
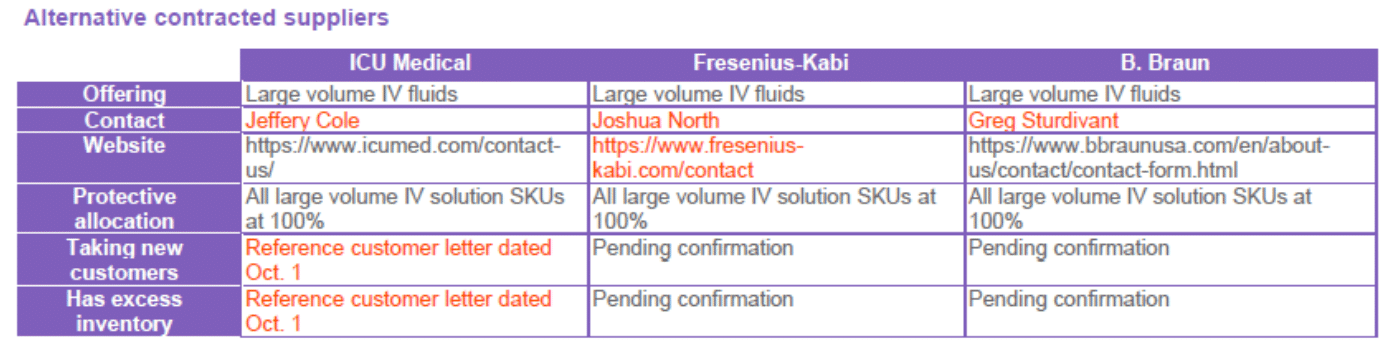
Alternative Contracted Suppliers